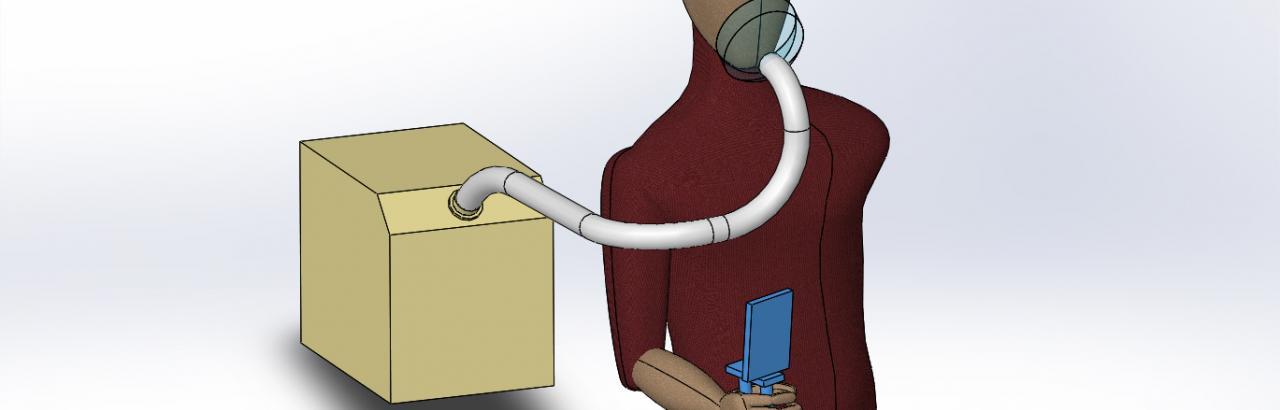
Objective
A study to investigate the effectiveness of daily acute intermittent hypoxia therapy (dAIH), coupled with a massed practice intervention or the use of high repetition training with the Rapael Glove, to improve upper-extremity function in individuals with chronic incomplete cervical SCI.
Who Can Participate
- History of spinal cord injury between the levels of C3-T1
- At least 6 months since the onset of injury.
- No history of heart problems.
- Not currently participating in another research study.
Compensation
If you agree to take part in this research study, we will pay you the following for for your time and effort:
- $40 for the initial screening visit
- $40 for overnight oximetry assessment
- $40 for each training session that you complete with intermittent hypoxia and/or training
- $40 for each baseline and follow-up assessment session
If you visit the Shirley Ryan AbilityLab but are not able to complete the session that day, you will be paid $20 for your time and travel expenses. We expect each training session to last no more than 4 hours. However, if the session goes beyond 4 hours or if your total travel cost exceeds the reimbursement provided, you will be paid $10 for each additional hour. If you travel more than 20 miles to visit the laboratory, then you will be provided an additional $20 for each session you attend.
Age Range
18-70